About clinical trials
Clinical trials are very demanding next to the clinical routine in hospitals. However, they also are drivers in translational research and drug development. Commitment and contribution of medical staff and patients are essential for steady progress in EB treatment.
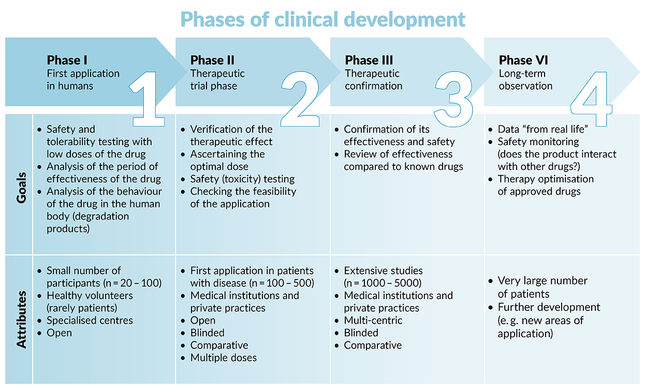
As of now, there is no causal systemic, long-lasting therapy for EB available. Until such is achieved, new approaches for the symptomatic treatment or local causal therapies will continue to be developed. They aim to increase the patient’s quality of life and correct the underlying gene defect locally in highly affected areas, respectively. In order to translate promising results from the lab to the clinics (“Bench-2-Bedside”), clinical trials are conducted. Depending on the trial phase (phase I-IV), the main focus is on safety assurance, dosage finding, efficacy testing, and long-term tolerance observations (see figure below).
Interventional studies
Commonly referred to as clinical trials, interventional studies are prospective studies tailored to evaluate the direct impact of treatment or preventative measures in EB patients. This can involve a potential drug, medical device, activity, or procedure.
New potential drugs need to pass through three phases (1, 2, 3) of interventional testing to show that they are safe and effective before receiving approval from the U.S. Food and Drug Administration (FDA) or European Medicines Agency (EMA). After a drug is approved, further studies (phase 4) will be performed to monitor for safety and effectiveness.
Observational studies
Besides testing new therapy approaches for EB patients in clinical trials, observational studies, in which researchers observe participants and track health outcomes over time, are an important category of study designs, to address investigative questions. Well-designed observational cohort studies in EB are helpful in evaluating clinical and epidemiologic characteristics, phenotype-genotype correlations, natural history of the disease, molecular or cellular signatures, impact of treatment strategies and secondary outcomes of clinical studies. Further, basic science studies utilize hypothesis-driven experimental designs to improve the knowledge and understanding of underlying disease mechanisms in EB.
The aim of a clinical trial is market authorization and therefore, the availability of effective therapies for patients. To achieve this high aim a large number of participants are needed. The patient cohort in an orphan disease such as EB is small, hence the demands for multicentric, international trials. Here EB Clinet can be of help in facilitating communication and connection of clinicians and expertise.
To be able to handle the increased time, administrative and financial effort during clinical trials, a well-trained team of physicians, nurses, and study coordinators are needed. Before the inclusion of patients, competent authorities and an ethics committee must release all study material (study protocol, informed consent forms, investigator medicinal product dossier, questionnaires, and information material). Eligible patients (defined by inclusion and exclusion criteria) must be compliant with the study protocol. Each intervention and examination has to be documented in detail following Good Clinical Practice. After completion, the trial results are evaluated and statistically analyzed and should be published.
Database for EB clinical trials
EB Resnet members prepared a database summarising all interventional and observational studies involving EB, that are registered on Clinicaltrials.gov, the EU Clinical Trials Register, the International Clinical Trials Registry Platform (ICTRP), and the UMIN Clinical Trials Registry.